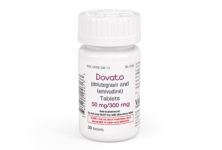
The US Food and Drug Administration (FDA) and the European Medicines Agency’s (EMA) are recommending new safety precautions for ViiV Healthcare’s HIV drug dolutegravir.
Dolutegravir is the active ingredient in three of ViiV’s products, Tivicay (dolutegravir) and two fixed-dose combination drugs, Juluca (dolutegravir/rilpivirine) and Triumeq (abacavir/dolutegravir/lamivudine).
The recommendations are based on the preliminary findings of a large observational study of more than 11,000 HIV-infected women in Botswana that found a higher rate of neural tube defects in infants born to mothers taking dolutegravir at the time of pregnancy or who began taking it during the first trimester.
The study found that 4 of the 426 (0.9%) infants whose mothers took dolutegravir at the start of or in the first trimester of pregnancy were born with a neural tube defect, compared to 14 of the 11,173 (0.1%) infants whose mothers took other HIV medicines.
Both regulators say they are looking into the preliminary results, though final results from the study are not expected to be available until next year.
As a precaution, both regulators are recommending that women who may become pregnant consult with their physicians before starting on a dolutegravir-containing drug and recommend that women of childbearing age taking the drug use contraceptives. Additionally, both regulators say that women of childbearing age should take a pregnancy test before starting on dolutegravir.
However, both regulators caution that women should not stop taking dolutegravir without consulting with their physicians as discontinuing the drug without being prescribed a suitable alternative treatment could cause their HIV infection to worsen.
New Safety Recommendations for Esmya
EMA’s Pharmacovigilance and Risk Assessment Committee (PRAC) also issued new safety recommendations for Gedeon Richter’s Esmya (ulipristal acetate) after completing its safety review of the drug.
In February, PRAC recommended that women taking Esmya to treat uterine fibroids undergo regular liver testing and that patients who have already completed a course of the drug hold off on beginning a new course while the agency investigated reports of liver failure related to the drug.
Based on its review, PRAC says Esmya “may have contributed to the development of some cases of serious liver injury,” and says the drug should not be prescribed to women with known liver problems.
PRAC also recommends that women take a liver function test before beginning on Esmya, then once a month during the first two courses of treatment and again two to four weeks after stopping treatment.
Additionally, PRAC says only women who are not eligible for surgery should take more than one course of the drug and is recommending that boxes of the drug include a card informing patients of the need for liver monitoring and advising them of symptoms of liver injury.
PRAC’s recommendations are being sent to EMA’s Committee for Medicinal Products for Human Use (CHMP) for a final opinion before being passed on to the European Commission for final legal action.


 ПОИСК ПО САЙТУ
ПОИСК ПО САЙТУ  поиск по ресурсному центру
поиск по ресурсному центру